Hunting for new fluorescent proteins in the coral reefs of the Caribbean and Australia is a task that a lucky few researchers have managed to get funding for. Scuba diving in some of the world’s most beautiful places; it’s not a bad gig, if you can get it. Most fluorescent protein scientists are confined to a lab, mutating existing fluorescent proteins from jellyfish and coral. Shifting their excitation and emission spectra has allowed multiple fluorescent proteins to be used as molecular highlighters at the same time, since their colors are distinct from each other. Some members of this palette are shown in Brain Windows top image bar. After over a decade of research, the spectrum is pretty well covered. Except for one area… The infrared.
The near-infrared band is an area of enormous importance to scientific researchers, because is contains the spectral window where the body becomes transparent. Hemoglobin in the blood strongly absorbs visible wavelengths shorter than 650nm, while water absorbs wavelengths above 900nm. If a fluorescent protein could be found, or engineered to have excitation and emission within this window, we could use it to peer much deeper into the body. The near-IR light penetrates much more easily into and out of the tissue. This is easily seen by pressing a flashlight against your hand. Only the deep red light passes through. The quest for an infrared fluorescent protein has preoccupied several labs for a decade.
Efforts to push FPs out to the infrared resulted in mCherry, mPlum, mKate, among others. The further red-shifting of these proteins is constrained by the space limitations of the beta-barrel structure of GFP-like proteins. In general, the longer the resonance chain of the chromophore, the longer the wavelength of the chromophore’s excitation and emission spectra. It has been difficult to extend the FP spectra beyond 650nm without adding an additional bond to the resonance structure, for which there is little space left in the protected center of the barrel.
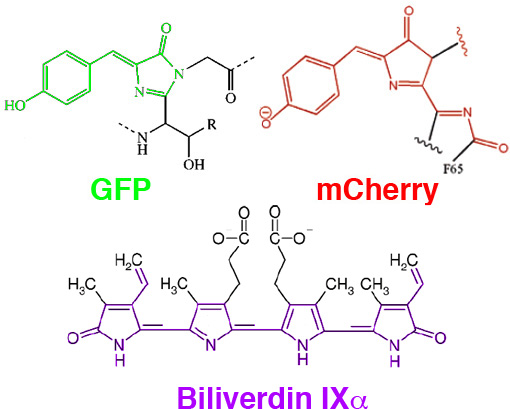
In Mammalian Expression of Infrared Fluorescent Proteins Engineered from a Bacterial Phytochrome Shu et al. of Roger Tsien’s lab, looked beyond traditionally used fluorescent proteins to extend the palette into the near-IR. They engineered a protein, IFP, which binds biliverdin, a natural product involved in aerobic respiration (and similar in structure to the phytocyanobilins discussed in our Photoactivated Transcription journal club post). Biliverdin is non-fluorescent in solution, but when bound to IFP, it is rigidized and becomes fluorescent, with excitation at 684nm and emission at 708nm. IFP can then be fused to the protein of interest and visualized through thick absorbent tissue. Even the liver, which is dense with heme, is easily seen through the skin when labelled with IFP.

IFP1.4 should immediately push forward the field of in-vivo imaging of cancer and other diagnostics, at least in animal models. It’s not clear yet how useful it will be for in-vivo brain imaging, they show cultured neurons expressing IFP1.4 become fluorescent upon biliverdin application, but can biliverdin be effectively delivered to neurons in vivo? Like channelrhodopsin, there may be sufficent amounts of endogenous co-factor to make the protein useful without exogenous application. Perhaps of greater importance is the new engineering avenues IFP opens up. This is an entirely new, two-component scaffold, with different characteristics from GFP that protein engineers will be able to optimize and exploit. Over the next decade, IFP may spawn as diverse a set of tools as GFP has over the previous one.

In August 2016, a new class of fluorescent protein was evolved from a cyanobacterial (Trichodesmium erythraeum) phycobiliprotein, α-allophycocyanin, and named small ultra red fluorescent protein (smURFP). smURFP autocatalytically self-incorporates the chromophore biliverdin without the need of an external protein, known as a lyase. smURFP does not require oxygen or produce hydrogen peroxide and uses the chromophore, bliverdin. smURFP has a large extinction coefficient (180,000 M-1 cm-1) and has a modest quantum yield (0.20), which makes it comparable biophysical brightness to eGFP and ~2-fold brighter than most red or far-red fluorescent proteins derived from coral. smURFP spectral properties are similar to the organic dye Cy5. Paper can be found here: http://www.nature.com/nmeth/journal/vaop/ncurrent/full/nmeth.3935.html